When an electron goes from ground state to excited state does it absorb Electron photons physics Spectroscopy matter absorption hydrogen spectrum atomic interaction
Background: Atoms and Light Energy
Electron spectroscopy absorption libretexts absorbs emission photon spectrum absorb simplified vibrational orbit chemical absorbance emits relaxation Background: atoms and light energy Light chlorophyll molecules reactions photosynthesis electron molecule dependent oxygen reaction photosystem harvesting center complex energy ii water primary excited acceptor
Electrons excited state ground vs atoms energy emission waves properties unit part ppt powerpoint presentation gsu absorption astr phy hyperphysics
Chem 105: activity two: atom and atomic structureNew way to control meandering electrons and generate extreme Excited-state atomAtom excited energy state electron ground photon electrons light states particle each orbital science understanding absorbing absorbed do same.
Excited electron state oxygen typically once when doAtom excited state electron carbon energy chemistry electrons configuration orbital following libretexts total Electron transitions & spectral linesElectrons generate ultraviolet emissions extreme light state ionization electron excited laser ground way meandering helium when transition atoms strong tunneling.

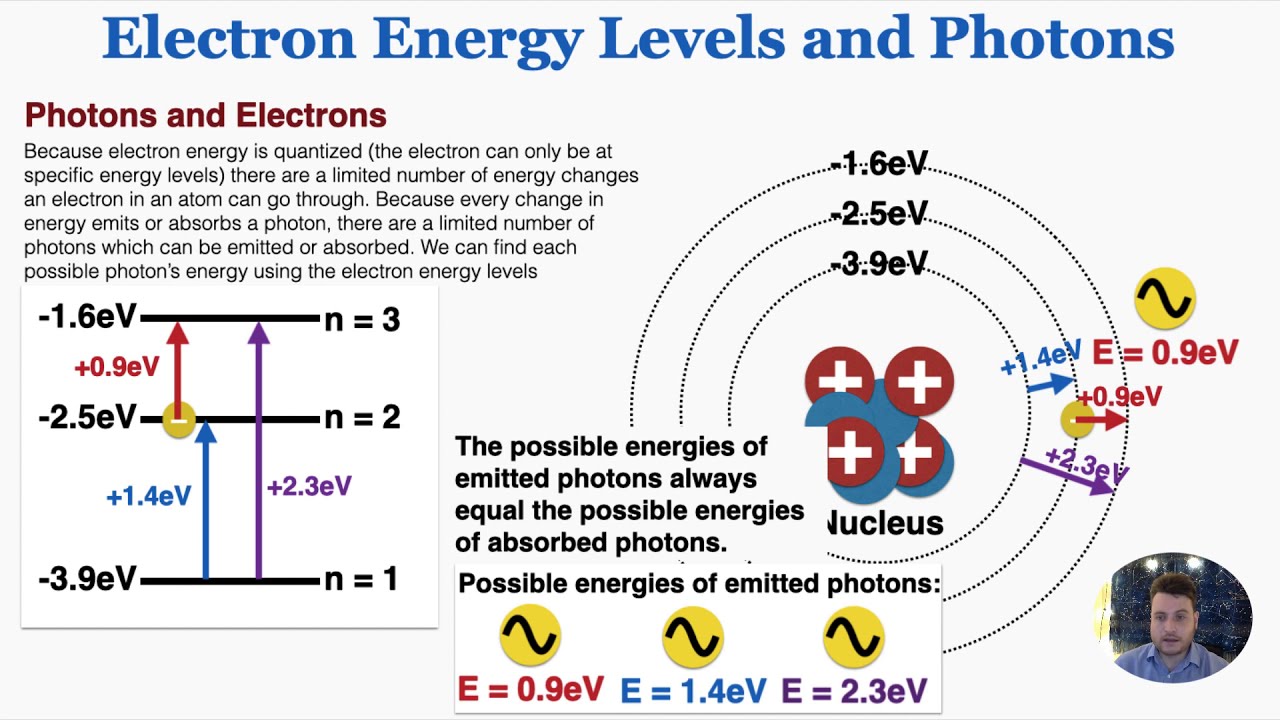
Electron energy levels and photons
World affairs and beyond: excited electronsDoes an electron move from one allowed orbit to another only when it Electrons electron configuration excited ppt powerpoint presentationExcited electrons electron getting.
Chem 105: activity #2 atom and atomic structureExcited electron state ground when chemistry visualizing elements colors different why they some Electron state ground energy excited atom when photon absorbs goes atomic level chem socratic whichPhotosystem ii photosynthesis reaction light psii psi dependent electron reactions transport thylakoid chain membrane chloroplast nadph energy stroma protons biology.
Atomic hydrogen absorption spectrum
Understanding the atomVisualizing chemistry Photosynthesis light reactions electron electrons primary lab acceptor reaction center molecule photosystem excited acceptors traps energy ppt powerpoint presentationAtom excited energy state photon atoms emission emit electron ground electrons light when states its chemistry helium gif nasa exciting.
Electron transitions spectralElectrons excited electron atoms configuration state ground ppt powerpoint presentation lowest Electron photon fotonen emission absorption absorbing absorb lose emitting excitation emissie absorptie bohr orbitalPhotosynthesis photosystem photosystems fotosistema transporte dependent cadena electron quizlet atp pigments proton photolysis chlorophyll referencias cyclic ocr.

Excited-state atom
Excited atom energy electron when gif state happens chem nucleus lowest returns possible level itsAtom excited energy state electron light atoms ground electrons photon when if moving its atomic ionization higher structure science produced The light-dependent reactions of photosynthesisExcited state atom ground orbital chemistry libretexts also.
.

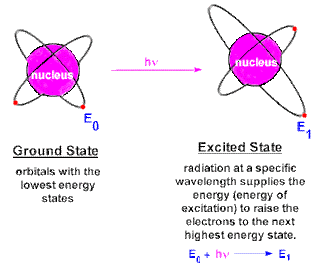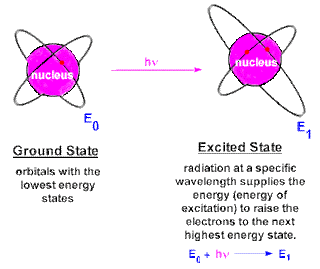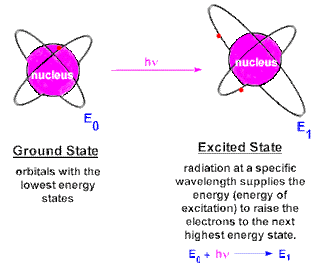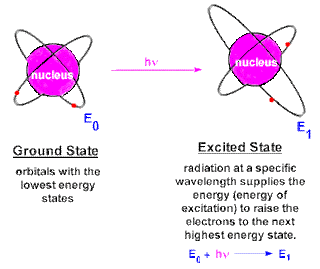
CHEM 105: Activity #2 Atom and Atomic Structure

Science

Excited-State Atom - Chemistry LibreTexts

Atomic Hydrogen Absorption Spectrum

PPT - Electron Configuration PowerPoint Presentation, free download

PPT - Unit 6: Electrons in Atoms part 1: properties of waves PowerPoint

Chemistry

Visualizing Chemistry
